Learn vocabulary terms and more with flashcards games and other study tools. The reason why theres an extra proton H with ammonia is because due to the fact that it is highly polar it will attract a hydrogen atom of a water molecule which it has dissolved in.
Why Life Depends On Water Biology For Non Majors I
That is because some bonds are broken that kept the.

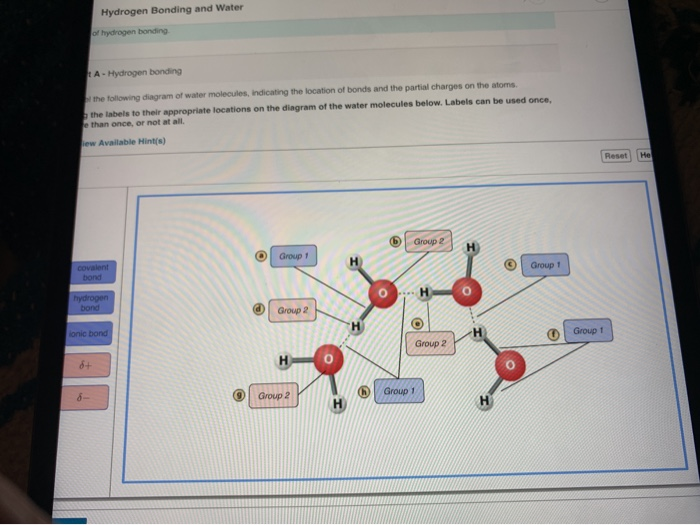
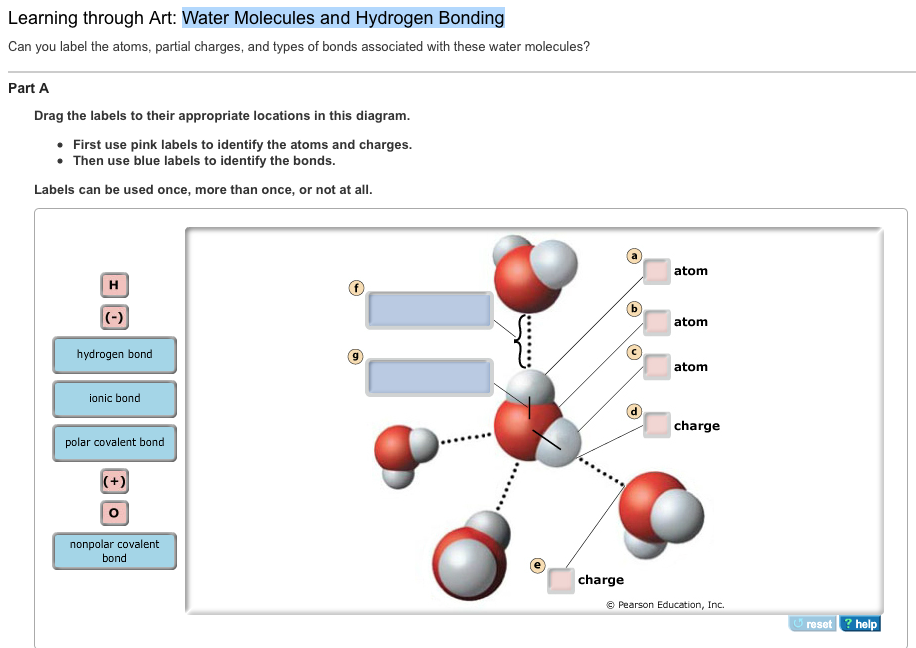
. Use that information to draw a diagram of a water molecule that shows electron sharing between the single oxygen and the two hydrogens. Ag the labels to their appropriate locations on the figure. Can you label the atoms partial charges and types of bonds associated with these water molecules.
Oxygen has 8 protons 8 neutrons and 8 electrons. Waters chemical formula is H 2 O. This allows it to be the solvent of life.
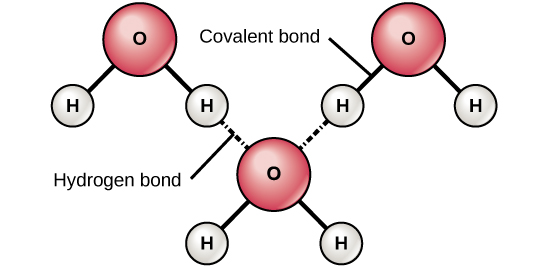
Learn vocabulary terms and more with flashcards games and other study tools. The figure below shows how the bent shape and two hydrogen atoms per molecule allows each water molecule to be able to hydrogen bond to two other molecules. Sharing of electrons between outer orbitals of adjacent atoms.
Many organic carboxylic acids form hydrogen-bonded dimers in the solid state. Ammonia mp 78 bp 33C is hydrogen-bonded in the liquid and solid states. The molecules are stuck in the solid state ice.
All of the electron pairsshared and unsharedrepel each other. They use heat energy to break the attractive forces bonds. Online interactive water molecule animation.
Solved Learning Through Art Water Molecules And Hydrogen Chegg Com A An illustration of how the covalency competition between tetrahedral and octahedral cations in overlapping with oxygen p orbitals M T M O competition results in an asymmetrical M T O. Use an online lab to experiment with water molecules. Up to 256 cash back Get the detailed answer.
Its unique ability to attract an exceptionally large number of hydrogen bonds induces the formation of a dense hydrogen. In water the attraction between a slightly positive hydrogen atom of one water molecule and the slightly negative. This creates a high surface tension of water.
A water molecule consists of two hydrogen atoms bonded to an oxygen atom and its overall structure is bent. Water readily sticks to many other substances a. The Hydrogen Bond and the Water Molecule offers a synthesis of what is known and currently being researched on the topic of hydrogen bonds and water molecules.
Sets found in the same folder. First use pink labels to identify the atoms and charges. Students work through a cognitive conflict exercise a simple experiment and then carry out research on a material containing hydrogen bonds.
As heat is added solid melts to be liquid which is more flexible sloshes about. Aqueous solution and form a fourth hydrogen bond with it. Here the hydrogen bonding acceptor is the electron cloud of a benzene ring.
Water molecules contain two hydrogen atoms pictured in green bonded to one oxygen atom blue. Learning through art water molecules and hydrogen bonding LIMITED TIME OFFER. View the full answer.
The electrons within a water atom spend much more time circling the oxygen atom than the hydrogen atoms. Students will understand that hydrogen bonds. Hydrogen has one proton and one electron.
For example in water molecules H 2 O hydrogen is covalently bonded to the more electronegative oxygen atom. Therefore hydrogen bonding arises in water molecules due to the dipole-dipole interactions between the hydrogen atom of one water molecule and the oxygen atom of another H 2 O molecule. GET 20 OFF GRADE YEARLY SUBSCRIPTION.
Learning through art water molecules and hydrogen bonding. Interaction through space between two charged atoms. This is a space-filling ball diagram of the interactions between separate water molecules.
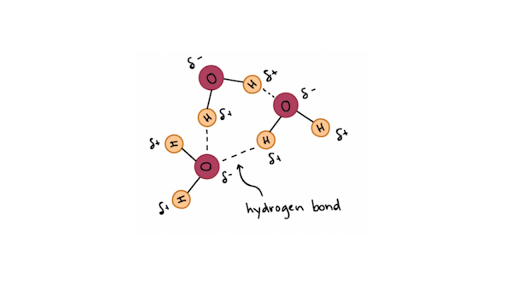
Its oxygen atom has a slightly negative charge and its hydrogen atoms each have a slightly positive charge. Molecules 02 Post Lecture alkthrough. Indeed water as found in nature.
When water molecules from hydrogen bond with other molecules the process is called adhesion. The most simple water molecular H2O is a fascinating but poorly understood molecule. The large oxygen atom has a stronger attraction for electrons than the small hydrogen atoms.
When you have all. Hydrogen Bonding in Water art A e figure shows how water forms hydrogen bonds. Terms in this set 11.
Hydrogen bonding is responsible for ammonia s remarkably high solubility in water. Water has an amazing ability to adhere stick to itself and to other substancesIn the case of water hydrogen bonds form between neighboring hydrogen and oxygen atoms of adjacent water molecules. Learning through art water molecules and hydrogen bonding OneClass.
1 Water the molecule. The oxygen atom is far more electronegative than the hydrogen atoms. Polar molecules do not share their electrons equally.
Because of the strong hydrogen bonds water molecules are able to stay condensed in the liquid state. Water is made of three atoms one oxygen and two hydrogens represented by the chemical formula H 2 O. Then draw the structural formula.
What is a covalent bond. Basic chemical structure of a water molecule. Exploring polarity with paper molecules Allow 10-15 minutes prep time 30 minutes for the video Click on the first link to download and print the pages you will need for this activity.
Water also forms hydrogen bonds with itself called. Hydrogen bonding BETWEEN water molecules. Hydrogen Bond Interactions Between Water Molecules in Bulk Liquid Near Electrode Surfaces and Around Ions.
This is because the oxygen atom in addition to forming bonds with the hydrogen atoms also carries two pairs of unshared electrons. Because the oxygen atom has a stronger pull on the negative bonding electrons the oxygen atom has a slightly negative charge and hydrogen atom a positive charge. The attraction between individual water molecules creates a bond known as a hydrog.
These activities introduce hydrogen bonds as intermolecular bonds made between specific permanent dipoles. Answer 1 of 2. Reset Help I Hydrogen Slightly negative charge Water molecule Oxygen Slightly positive.
Distance between two oxygen atoms lies between 025-035 nm H-bond distance lies between 015-025 nm and the angle of the O-H. Xenides et al 2006 have suggested the following parameters for H-bonded water molecules. The most stable arrangement is the.
Most molecular compounds that have a mass similar to water are gases at room temperature. Protein structure amino acids. Answer 1 of 2.

Why Is H2o Liquid And H2s Gas At Room Temperature Quora

Mastering Biology 2 Flashcards Quizlet
Solved Hydrogen Bonding And Water Of Hydrogen Bonding A Chegg Com

Hydrogen Bond Between Water Molecules Diagram Quizlet

How Is Hydrogen Bonding Among Water Molecules Related To The Structure Of The Water Molecule Socratic Water Molecule Chemistry Education Teaching Chemistry

Chapter 3 Water And Life Flashcards Quizlet
Solved Can You Label The Atoms Partial Charges And Types Chegg Com
0 comments
Post a Comment